Manufacturing & Materials for Next-Generation Batteries
| Thermal Stability of Li-ion Battery Cathode Materials | From the early adoption of Li-ion battery systems in portable electronics, thermal runaway incidents have always been a major concern. In general, the thermal runaway is the outcome of a chain of exothermic reactions, that result in oxygen release from the positive oxide cathode. The released O2 eventually reacts with the decomposed organic electrolyte in a self-progressive and uncontrolled exothermic reaction and can lead to the ignition of the battery. We utilize in-situ transmission electron microscopy to better understand the O2-release phenomena and the thermal instability of the positive oxide cathodes. Subsequently, based on the acquired understanding, we exploit 2D materials to suppress this detrimental reaction and improve the thermal stability of layered oxide cathodes. |
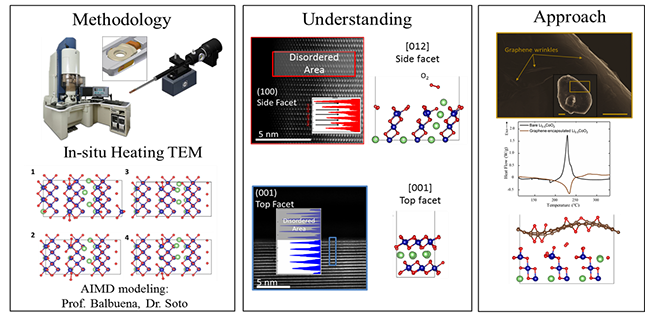
|
| Dendrite Growth Mitigation in Lithium-metal Batteries | Lithium (Li) metal is an ideal anode material for Lithium batteries due to its ultra-high theoretical capacity and low redox potential. Of all the approaches proposed to prevent dendrite formation, constructing a stable interface between Li metal and electrolyte has gained extensive interest. We use two-dimensional (2D) materials such as graphene oxide to effectively regulate the Li deposition. Our electrochemical measurements illustrate ~100% improvement in the cycleability of Li-metal anode through coating of Graphene Oxide nanosheets (GOn) on the surface of the separator. Ab-initio molecular dynamics (AIMD) simulations suggest that Li-ions initially get absorbed to the lithiophilic GOn flakes, and diffuse through defect sites by knock-off mechanism. This delayed Li transfer eliminates the "tip effect" leading to a more homogeneous Li deposition. Also, phase-field modeling (PFM) demonstrate that GOn have sufficient mechanical rigidity to block the anisotropic growth of Li in the direction of applied electric field, impeding the dendritic Li growth. |
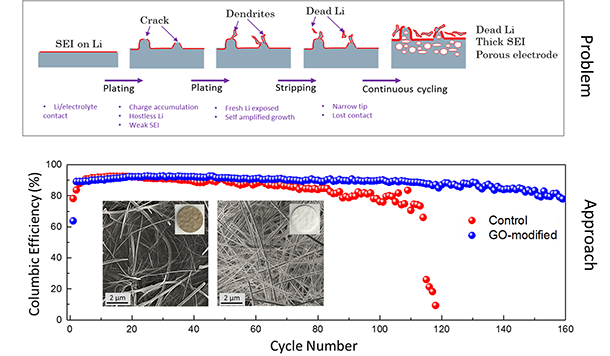
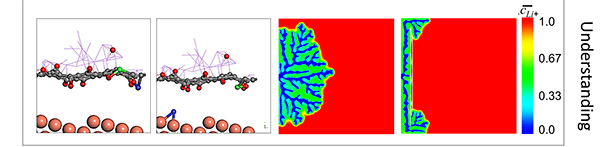
|
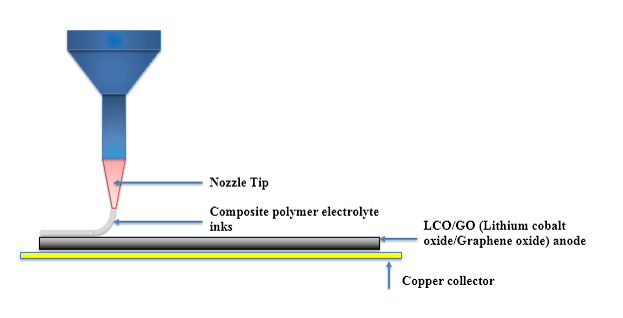
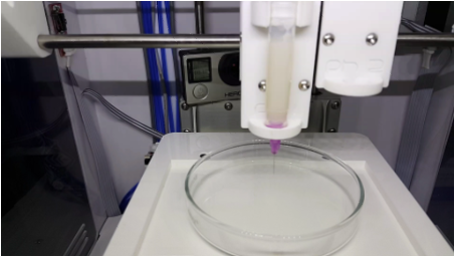
| 3D Printing of Polymer Electrolytes for Li-ion Batteries |
Direct Ink Writing(DIW) is one of 3D printing technologies drawing worldwide attention due to simple printing mechanisms and low-cost fabrication processes. Moreover, Direct Ink Writing has a broad diversity of materials selection, length scale and outstanding structure finish. Ink design is commonly considered as one of the most essential parts of direct ink writing, because the three-dimensional architectures challenge the self-supporting features of inks. 3D printed batteries present a means through which we could overcome this challenge, and enable integration of the battery directly into the electronics fabrication process. This would be fully utilizing the limited space available.
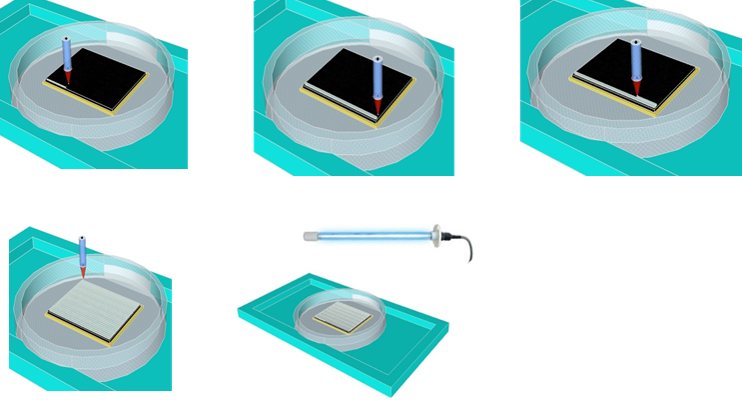
|
|
| Solid/Polymer Electrolytes for All-solid-state Batteries |
Polymer-based electrolytes
Inorganic fillers were added to study the influence on the performance (electrochemical & mechanical) of the electrolyte. Solid state NMR, FTIR, Raman, XRD, TGA tests are conducted to study the mechanisms behind filler enhanced polymer electrolyte. Ceramic-based electrolyte Oxide-based high-ionic conductivity materials such as LLTO, LLZO, LATP are synthesized through different strategies. Atomic resolution studies and Scanning Transmission Electron Microscopy are used to investigate their ion transport mechanism, failure mechanism etc. Thin film batteries Thin-film batteries are fabricated by Focused Ion Beam method and the effects of biasing, heating, and cooling are studied using In-situ Transmission Electron Microscopy. |

|
| Electrolytes for Magnesium-ion Batteries | Multivalent-ion batteries offer the promise of at least 1.5 - 2 times the energy density of monovalent-ion systems such as Li-ion and Na-ion batteries. Magnesium battery systems are of particular interest owing to the dendrite-free deposition, and high stability of magnesium metal in air and moisture. However, there are significant challenges that have been hindering the development of high energy-density magnesium ion batteries, among which the electrolyte development is deemed as an important one. Research progress on magnesium intercalation (cathode) materials have also been limited by the availability of electrolytes that have both a high oxidative stability, and a good compatibility with Mg metal anode. We aim to address these issues and synthesize a Mg-battery electrolyte with high cyclability. |

|
In-situ TEM for Nanomaterials/Biomaterials Research
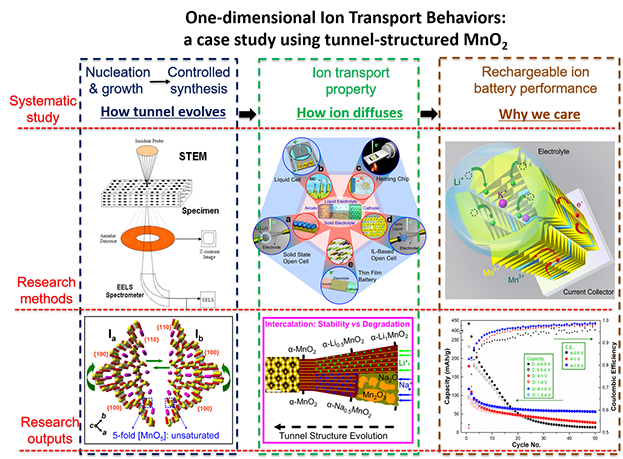
| Atomistic Understanding of Electrochemical Processes | In-depth understanding of diffusion dynamics of various charge carriers inside one-dimensional tunnel-structured transitional metal oxides, which represent a large family of functional materials in nature. A prototyped MnO2 tunnel structure has been targeted, and a systematic atomic-level study has been carried out in terms of tunnel evolution process, ion diffusion properties inside the tunnel, and the practical applications rooted on ion transport. |
|
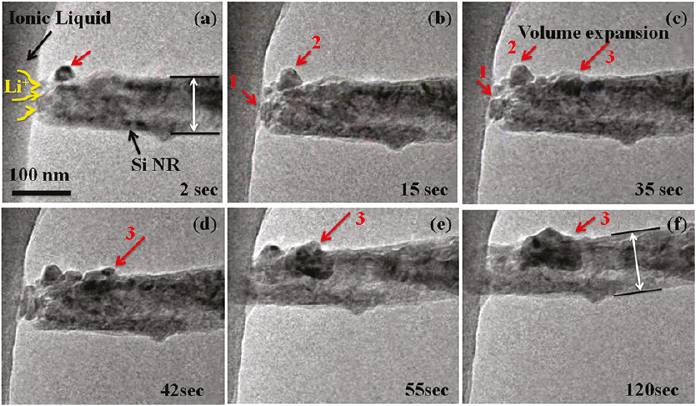
| In-Situ Electrochemistry in TEM for Li-ion & Na-ion Batteries |
The research objective here is to advance the understanding of the underlying mechanisms that are active in Li-ion / Na-ion reactions in electrodes and chemically-induced failure and fracture in Li-ion / Na-ion batteries. For this purpose, the cathode electrode will be subjected to electrochemical probing inside a transmission electron microscope (TEM) where the microstructure of electrodes can be simultaneously monitored in high resolution.
Room-temperature rechargeable Na-ion batteries can be competitive to Li-ion batteries due to their lower cost and the widespread availability of the sodium resources. The challenges of Na-ion batteries are much more complicated than the Li-ion case, as Na-ions are larger than Li-ions by 70%, which induces large mechanical stresses upon driving the Na-ions into the host electrode. The entry of sodium into the electrodes triggers complicated chemical reactions and failures that are poorly understood, and the battery life is reduced drastically |
|
| Investigation of Noble Metal Nanoparticles using In-situ Liquid Cell TEM | Noble metal nanoparticles with high surface area, suitable orientation and strong resistant to aggregation is of highly demand in design of next generation catalysts. Two-dimensional (2D) materials, a promising substrate for noble metal nanoparticles, provide a facile way to control particle size, orientation and prevent aggregation. However, little is known on how 2D substrates influence the nucleation and growth process of noble nanoparticles. In situ liquid and gas cell TEM, a power tool to investigating the effect of 2D substrates on the formation and stability of metal nanoparticles combined with atomic resolution HAADF-STEM imaging. The dynamics of nucleation, growth and coarsening can be observed in real time to develop methods to further control desired particle morphology. |
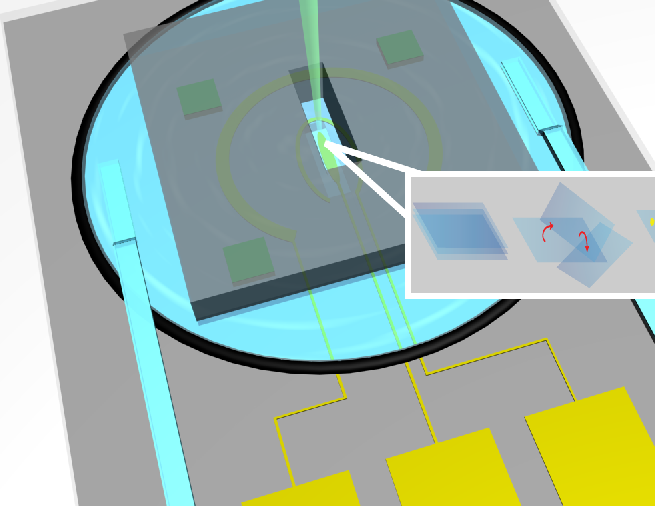
|
| Solvation and Nucleation processes in Minerals | We study the dynamic dissolution of gypsum (calcium sulfate dihydrate, CaSO4•2H2O) using in-situ Liquid cell TEM. We observed gypsum microparticles to gradually dissolve in water, forming sulfate (SO4)2- and calcium Ca2+ ions, which gradually saturate the surrounding aqueous phase. It is notable that the dissolve of gypsum particles prefer to proceed via the interfacial region, causing a crack to develop inside the gypsum flakes. At the later stage of the dissolving process, we observed the original gypsum flakes gradually to dissolve and fall apart to bonded needles. Further later, some interfaces were completely etched by water, and some needles were detached from the bounded ones. We also noted that the dissolution of the flakes or needles were not isotropic. |

|
| Properties of Materials in Liquid State using Graphene Liquid Cell |
GLC consists of a liquid sample encapsulated in between two graphene monolayers , which are electron-transparent and ion-impermeable.
Ferritins from various organs must be compared to understand the effect of differences in H and L subunits on the iron oxide mineral present in ferritins. Electron-matter interaction could be understood via Monte Carlo Simulation Technique Morphology changes via electron beam induced degradation in polymers must be imaged in liquid state so that dynamic transformation to a secondary material can be visualized. |
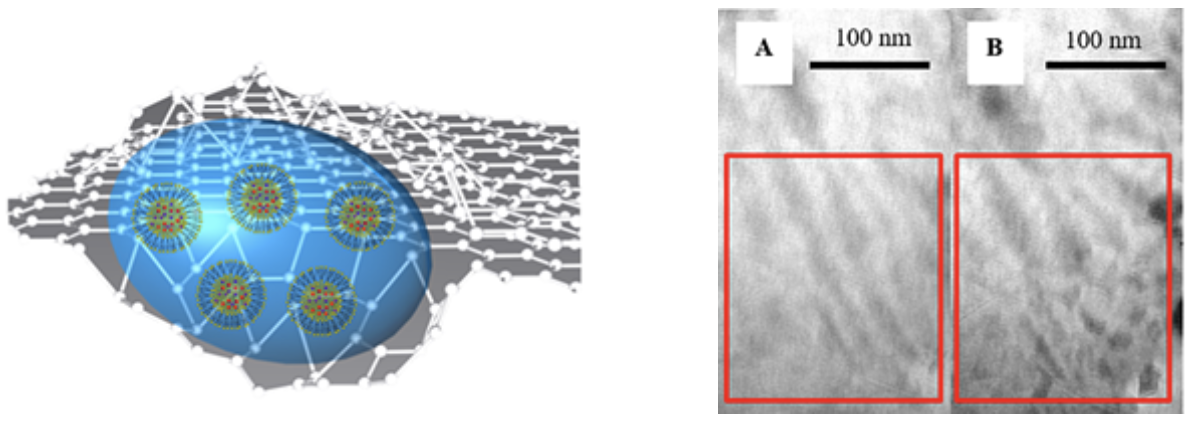
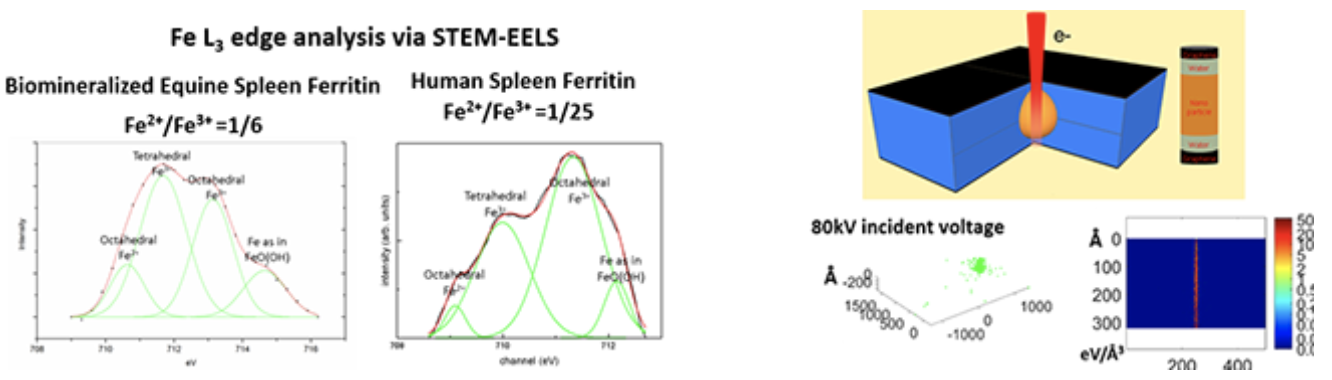
|
| Detection of Piezoelectric Current and Field Emission in ZnO Nanowires |
The detection of piezoelectric current in one-dimensional semiconductor materials has been a controversial issue due to the possibility of charge annihilation at nanoscale dimensions. We work on mechanically triggered electrical current in the uniaxially compressed individual ZnO nanobelts under no applied bias. The measurements are carried out in-situ in a transmission electron microscope. In contrast to the bending mode, the magnitude of the electrical current increases with the increase of uniaxial compression, which indicates load-mode dependency of the detected current.
The flow of electrical current through the ZnO nanobelts under applied stress can be explained based on the separation of ionic charges along the two ends of the nanobelts due to the applied compressive force. The charge separation is expected to induce an internal electric field inside the nanobelt and facilitates the free charge carriers to move through the nanobelt. Due to the separation and accumulation of charges, the metal-semiconductor system becomes forward biased when contact is established, leading to the flow of electrons through the Schottky barrier. |
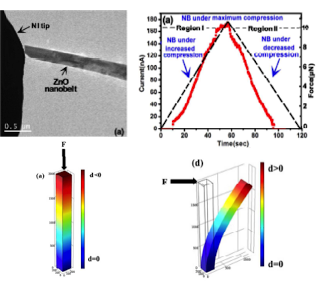
|
| Mechanical and Thermal Stability of Thermoelectric Nanowires |
One-dimensional nanostructures such as ZnTe, CdTe, Bi2Te3 and others have attracted much attention in recent years for their potential in thermoelectric devices among other applications. A better understanding of their mechanical properties is important for the design of devices. A combined experimental and computational approach has been used here to investigate the size effects on the Young’s modulus of ZnTe nanowires (NWs). The mechanical properties of individual ZnTe nanowires in a wide diameter range (50–230 nm) were experimentally measured inside a high resolution transmission electron microscope using an atomic force microscope probe with the ability to record in situ continuous force–displacement curves.
Link to article in Nanoscale Link to article in Journal of Applied Physics Link to article in Nanotechnology |
|